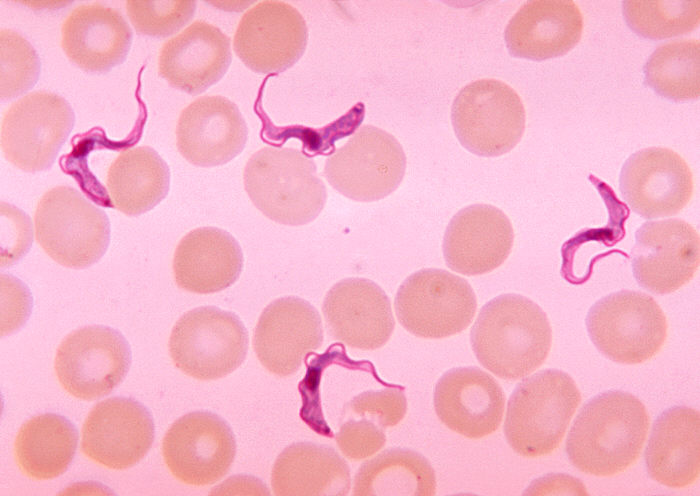
Trypanosoma brucei
About This Microbe: Jeff Lockwood
Overview
Trypanosomes (genus Trypanosoma) are single-celled, parasitic, flagellate protozoans. They move in a twisting, corkscrew-like motion (hence their name: trypano is Greek for borer and soma means body) via their flagellum. Rather counterintuitively, they lead with their tail-like flagellum (i.e., it does not propel them from behind but pulls them forward).
These creatures are heteroxenous, meaning that they must have more than one host to complete their life cycle, and most are transmitted to the bloodstream of a vertebrate by hematophagous (blood-feeding) insects, where they reside in the gut. Trypanosomes take on different forms in their various hosts. In general, the trypomastigote form occurs in the vertebrate’s extracellular environment and the amastigote form occurs in the intracellularly; the promastigote (or trypomastigote) form is found in the invertebrate.
Trypanosoma Brucei
Trypanosoma brucei causes sleeping sickness in humans, this protozoan causes nagana in cattle. Sleeping sickness (trypanosomiasis) is associated with extreme fatigue, achiness, and neurological symptoms (headaches, confusion, seizures, coma and death). Nagana causes fever, weakness, and lethargy, which lead to weight loss and anemia—and this disease may be fatal.
There are three subspecies: T. b. brucei (causes animal trypanosomiasis but doesn’t infect humans, so it is often used as a model in research), T. b. gambiense (causes slow onset, chronic trypanosomiasis; found in central and western Africa; humans are the primary reservoir) and T. b. rhodesiense (causes fast onset, acute trypanosomiasis; found in southern and eastern Africa; game animals and livestock are the primary reservoirs).
T. brucei is extremely rare in its capacity to cross the blood-brain barrier. Another remarkable feature of T. brucei is its capacity to avoid the host’s immune system. This ability is a function of its variable surface glycoprotein (VSG) coat. There are two mechanism involved. First, shielding is provided by the dense nature of the VSG coat which prevents the immune system from detecting the trypanosome’s plasma membrane. Second switching is the process by which the VSG coat undergoes frequent, random genetic modification at the time of cell division. In other words, the biochemical identity of the parasite changes in the course of an infection so that the host cannot mount an effective immunological response.
In the infection cycle, the trypanosome infects the midgut of the tsetse fly (procyclic life stage) and then migrates to the salivary glands (epimastigote life stage). There, some parasites undergo differentiation into the metacyclic life stage which is passed into the mammalian bloodstream during insect feeding. At this point some of the parasites adopt the “slender bloodstream life stage” while others undergo change to the “stumpy bloodstream life stage” which can then reinfect a tsetse fly upon feeding. The slender form can move the blood to infect the lymph and cerebrospinal fluids.
Scientists work
T. brucei was the organism that first I studied as an undergraduate and which formed the basis for my first scientific paper: Sanchez, G., J. Lockwood, and R. Chavez. Liver glycogen mobilization by Trypanosoma brucei sonicates. Comparative Biochemistry and Physiology 70B (1981): 447-450.
In this research, we found that T. brucei contained a glucose stimulating-substance (GSS) that could mimic the effect of the host’s glucagon, the hormone causing the conversion of glycogen (the storage form of glucose) into glucose. T. brucei feeds on carbohydrates (glucose) in the host’s bloodstream, and these substances are rapidly depleted causing hypoglycemia in the rat. Hence, it is to the parasite’s advantage to stimulate the host to convert stored forms of carbohydrates (glycogen) to free glucose. Both in vitro and in vivo studies using trypanosome sonicates (disrupted cells) showed that a substance in the parasite could induce an acute hyperglycemia, indicating that the liver cells of rats had been induced to release glucose. The upshot is that the trypanosome has evolved a chemical to “trick” the host into providing it with a continuous source of nutrients, until the host’s energetic stores are depleted.